Review the information presented in class today and complete the worksheet below:
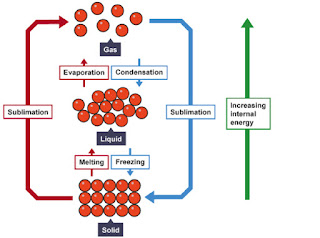
Fill in the phase changes in the blank provided.
Phase Change Worksheet
The graph was drawn from data collected as a substance was heated at a constant rate. Use the graph to answer the following questions.At point A, the beginning of observations, the substance exists in a solid state. Material in this phase has _______________ volume and _____________ shape. With each passing minute, _____________ is added to the substance. This causes the molecules of the substance to ____________ more rapidly which we detect by a ________________ rise in the substance. At point B, the temperature of the substance is ______°C. The solid begins to __________. At point C, the substance is completely ____________ or in a ___________ state. Material in this phase has _______________ volume and _____________ shape. The energy put to the substance between minutes 5 and 9 was used to convert the substance from a ___________ to a ___________. This heat energy is called the latent heat of fusion.Between 9 and 13 minutes, the added energy increases the ______________ of the substance. During the time frompoint D to point E, the liquid is ___________. By point E, the substance is completely in the __________ phase. Material in this phase has _____________ volume and ___________ shape. The energy put to the substance between minutes 13 and 18 converted the substance from a ___________ to a ___________ state. This heat energy is called the latent heat of vaporization. Beyond point E, the substance is still in the ______________ phase, but the molecules are moving _______________ as indicated by the increasing temperature.
Which of these three substances was likely used in this phase change experiment?
Substance
|
Melting point
|
Boiling point
|
Bolognium
|
20 °C
|
100 °C
|
Unobtainium
|
40 °C
|
140 °C
|
Foosium
|
70 °C
|
140 °C
|






No comments:
Post a Comment